Having the right perspective is key to understanding any type of problem. This post is intended to provide information about the manufacturing of many million doses of a novel mRNA vaccine. Just for the record, mRNA vaccines are indeed gene therapies.
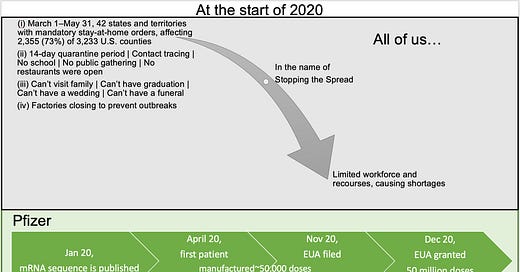
Remember… at the start of 2020, all of us were asked to put our selfishness aside, suspend our rights and act responsibly by being locked down in our homes … this is an important fact to keep in mind as you read along…
Given the circumstances, a question that should bother everyone of us and especially those who are involved in drug development is:
How were #PFE / BioNTech able to advance their mRNA vaccine and manufacture over 50 million doses in less than 12 months, while there were significant shortages of supplies and workforce?
This post will not be able to definitively answer this question, but will hopefully provoke inquisitive minds to explore possible answers …
Historical Overview of LNP Manufacturing
LNP technology is based on cell membranes largely composed of lipids - molecules contain one part that is water insoluble (hydrophobic) and another that is water soluble (hydrophilic). Such molecules can be dried as powders or oils, and can be easily rehydrated in water at the bedside whereby they self-assemble into higher-order spherical vesicles known as liposomes. [Stephen Allan, Evonik, 2020]
20 drugs that include LNP approved since 1995
Drugs mainly in oncology, i.e. LNPs are distributed through bloodstream
None were mRNA vaccines until Covid-19 vaccines were approved
Delivery systems involving siRNA-conjugates (not mRNA!) have drawn some interest, most therapies using LNP are non-viral formulations for gene therapies, cell targeting and other nanomedicines
2018 approval of Onpattro® for the treatment of polyneuropathy in people with the rare disease - small scale production of siRNA in LNP (Acuitas developed the LNP)
Key considerations for Formulation Development…
…It takes time to get the final formulation right
Functionality: the composition of the lipid components. For example, ionizable cationic lipids are generally responsible for maximizing the intracellular delivery of the nucleic acid and play a role in payload encapsulation. The length, level of unsaturation and linker moiety of the hydrocarbon chains and proton availability (pK values) for the lipid are other factors that can influence potency and efficacy.
Stability: to determine LNP functionality the particlse need to incorporate polyethylene glycol-lipids comprising a polymeric PEG chain and two hydrophobic lipid tails.
Quality of raw materials used to synthesize lipids, as well as the lipid type and source have a direct impact on the impurity profile, particle characteristics, particle stability, and final formulation release profile. If raw materials are not consistently good, formulation quality could vary.
Challenges with Lipid Nanoparticles (LNP) Manufacturing…
… It takes time to get the final manufacturing process right
LNP manufacturing - usually extrusion-based processes and though preferred for many liposomal formulations, are challenging with mRNA [Evonik, 2020]
Selecting the right manufacturing parameters is not trivial and requires experience with the different parameters [Roces et al., 2020]:
flow rate ratio (FRR) and total flow rate (TFR) significantly influence the physicochemical characteristics of the produced particles
increasing the total flow rate or increasing the flow rate ratio could lead to decrease in the particle size
Selecting the “ingredients” requires knowledge of how the components will interact with each other and with the mRNA [Roces et al., 2020]. Note: mRNA had not been encapsulated into LNPs for human use at the time of Covid-19 pandemic start. The characteristics of the final LNP are impacted by the amino lipid (cationic or ionisable); the buffer choice (citrate buffer pH 6 or TRIS pH 7.4); the type of nucleic acid payload (PolyA, ssDNA or mRNA)
Peek into the limited manufacturing information released for BNT162b2
Very limited manufacturing information has been made available thus far as part of the EUA, approved labeling and BLA documents released to the public. Based on my past experience, the Chemistry, Manufacturing and Controls (CMC) section of the BLA will be heavily redacted due to the perceived proprietary nature of the information such as (but not limited to) lot release and stability specifications and data, test methods and validations, formulation components and manufacturing processes, stability of the mRNA / LNP drug product and the diluent. It is the redacted portion that will be key for understanding who the product is manufactured and quality controlled. Thus far we know the following (excerpts from the EUA; no CMC information from the BLA has been released to date, not even the Table of Contents for the CMC portion):
In-process, release, and characterization data for a minimum of three process performance qualification (PPQ) drug substance (DS) batches for each DS manufacturing facility were provided.
The drug substance (DS, the attire ingredient preceding the final product) manufacturing process underwent changes during vaccine development; comprehensive analytical comparability assessment was performed and the submitted data support the comparability.
The manufacturing process for drug product (DP) was changed from a Classical process to an Upscale process involving an increase in batch size (capable of accommodating larger RNA input) to meet commercial need. A comparison of available DP batch release data and an in-depth analytical comparability assessment between six representative Classical process DP batches and one Upscale process DP batch supported the use of the Upscale process for DP manufacture under emergency use. A minimum of two (2) and more typically three (3) comparison batches were required.
Certificates of Analysis (CoAs) for a minimum of 3 GMP commercial-scale drug product lots from each manufacturing node were requested from the Sponsor to demonstrate process performance and consistency. Data from 4 manufacturing nodes were available during the EUA review. In addition, to support vaccine supply and availability, data from two additional nodes were to be submitted to the EUA between December 17 and December 23, 2020.
Once authorized, the Sponsor was to submit the CoAs of DP lots to be distributed under EUA for review at least 48 hours prior to lot distribution.
Stability studies were designed to support the use of vaccine under the EUA. All available stability data generated using the BNT162b2 active substance and drug product lots support the emergency deployment of the Pfizer-BioNTech COVID-19 Vaccine.
The analytical procedures developed and used for the release and stability monitoring were deemed appropriate and acceptable to be used for the control of active substance /drug product quality. All analytical procedures used for the release of emergency supply DS and DP have been adequately qualified.
For each of these facilities, FDA requested and reviewed information on equipment, facilities, quality systems and controls, container closure systems as well as other information.
It takes time to get it right …
… or does it?
Few final thoughts:
BNT162b2 uses commercially available lipids but also ‘proprietary, bespoke lipids’; ‘very exotic mixing technology’ to control the shape and composition of the lipid nanoparticles and mRNA; “Other drug companies cannot easily chip into the manufacturing due to the proprietary nature.” [www.chemistryworld.com, 2020].
All this creates manufacturing logistics challenges at a time of sparse resources due to lockdowns and quarantines.
ALC-0159 and ALC-0315 are novel and proprietary according to PFE/BioNTech BLA:
literature suggests reactions to these components and that they are highly inflammatory in mice (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8604799/pdf/main.pdf);
literature indicates they accumulate in the liver, spleen, adrenal glands and in ovaries, which is also seen in the BLA (Section 2.6.5)
PFE/BioNTech were able to set up 6 manufacturing sites between Q1 2020 and Dec 2020
The stability of the product was not specified in the EUA
Analytical procedures were not validated at time of EUA
Due to Covid-19 restrictions, there were no in person quality inspections
Based on the above information, it seems that it would take a considerable time to get the final product right in terms of formulation, testing, stability and scaled up manufacturing. In fact, examples of other vaccine development projects point to needing several years to accomplish the mission. However, in the case of BNT162b2/ Comirnaty all this was done in less than 12 months at the said scale while conducting nonclinical experiments and clinical studies in over 40,000 people worldwide.
… My professional curiosity is piqued …




Can you explain the storage and transport side. If you recall when these were released there was lots of hype about them needing to be stored and delivered at sub-freezing temps. Subsequently, read that they don't need to stored at sub freezing temps anymore. Seems odd such a significant change midway through rollout?
My layman's summary is, these shots were ready for deployment long before Covid? Or, they were produced in such a slipshod fashion that of course they failed? But if they were slipshod, why aren't they inert rather than dangerous? Thank you for your article.